Describe Where A Hydrogen Bond Can Form Among Water Molecules
So water has the formula h2o so the only possible h bonds that can form are between h and o within the structure or between the molecules of h2o so that water molecules are attracted to each other. The hydrogen bonds among water molecules are about as strong as those among ethanol molecules.
Water Is The Medium Of Life Ppt Download
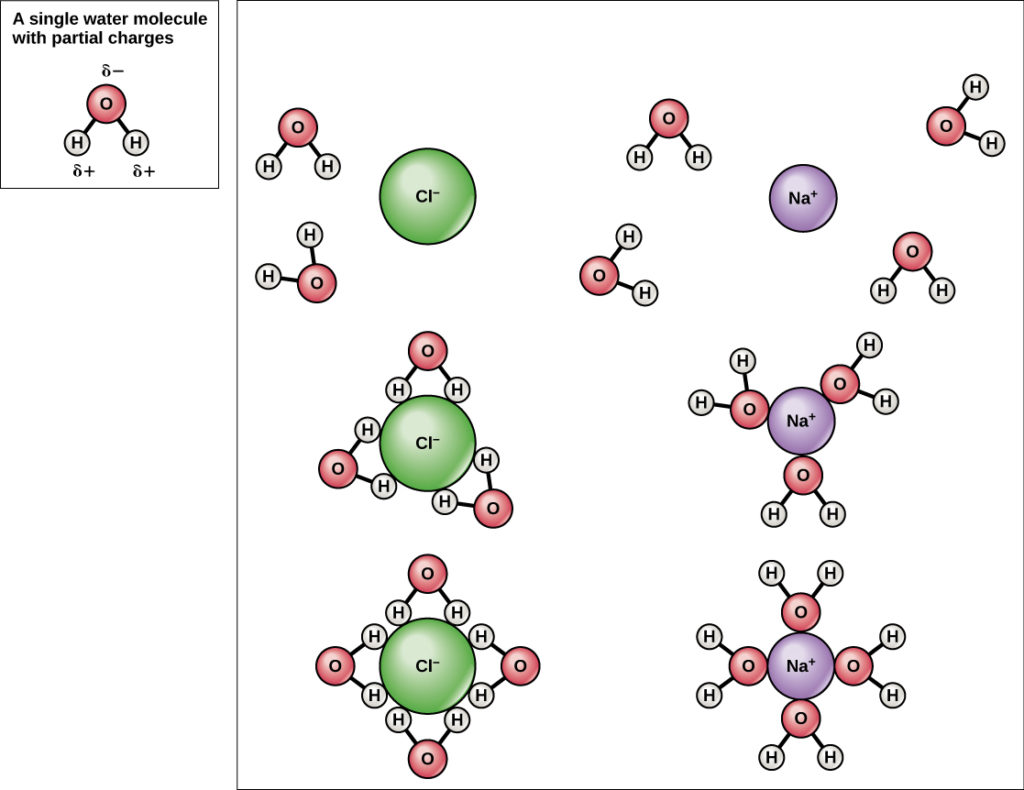
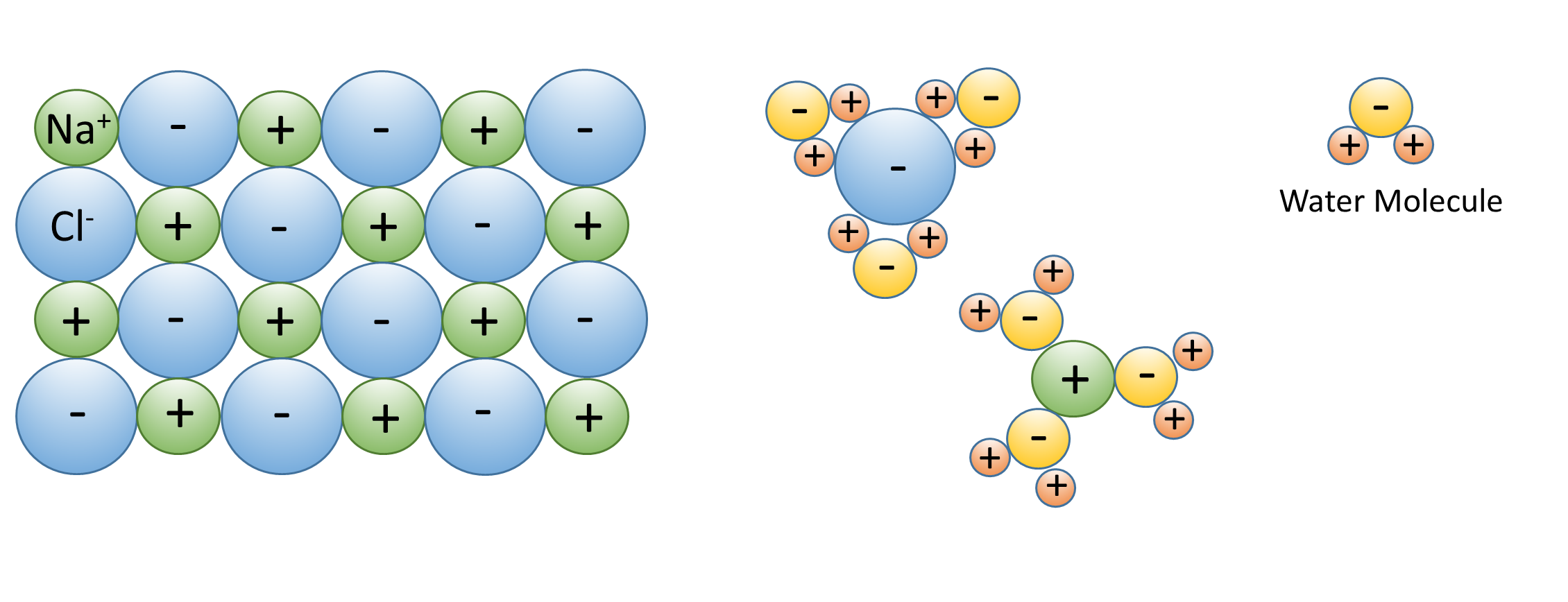
When an ionic or polar compound is exposed to water the water molecules surround it.
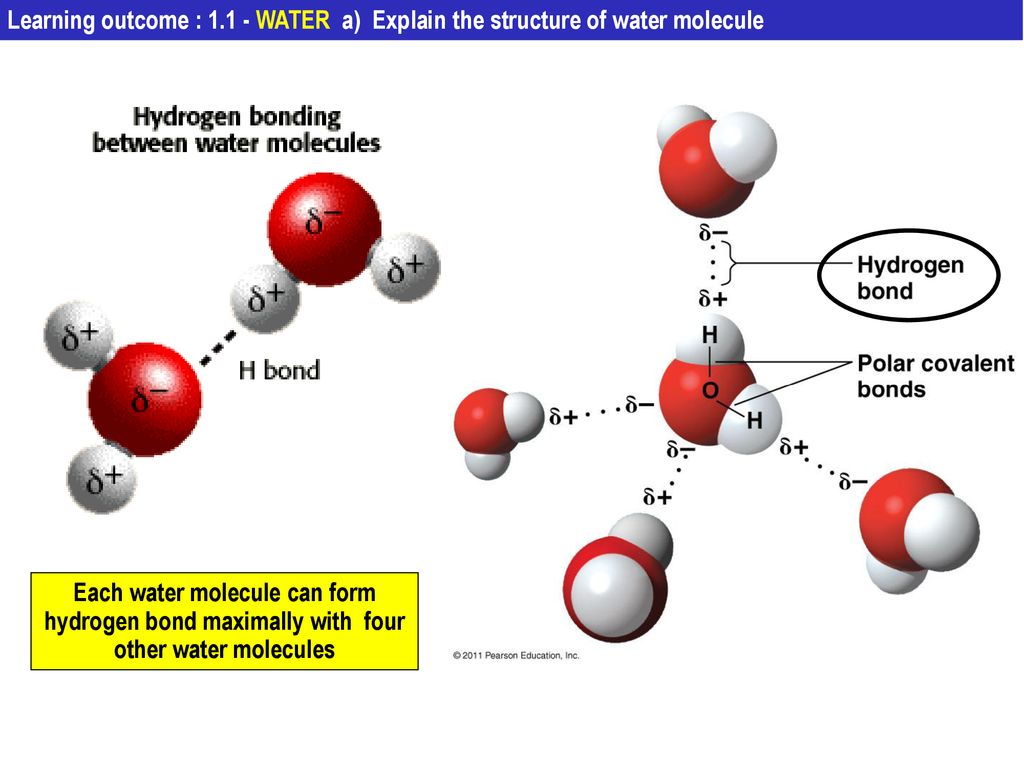
Describe where a hydrogen bond can form among water molecules. The molecule is slightly bent with oxygen in the middle and hydrogen on each end. A hydrogen bond forms in water molecules due to its shape. ø hydrogen bond is an electrostatic attraction between a hydrogen atom which is covalently bound to a high electronegative atom such as oxygen and nitrogen to another electronegative atom of same or different molecules of their close vicinity.
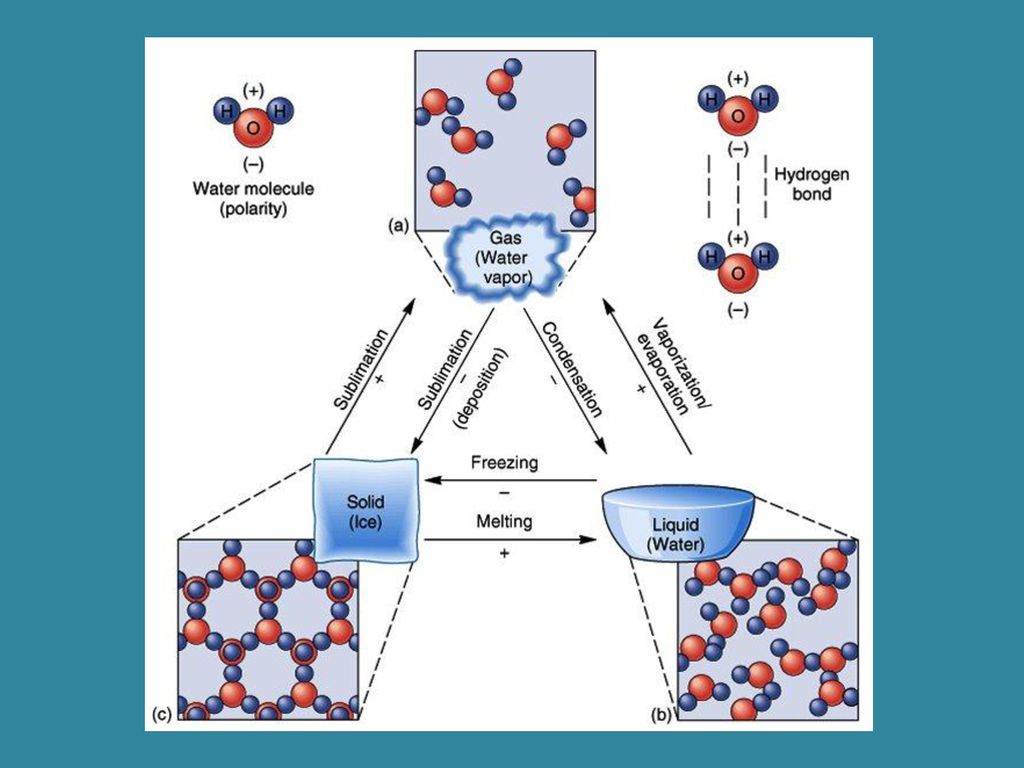
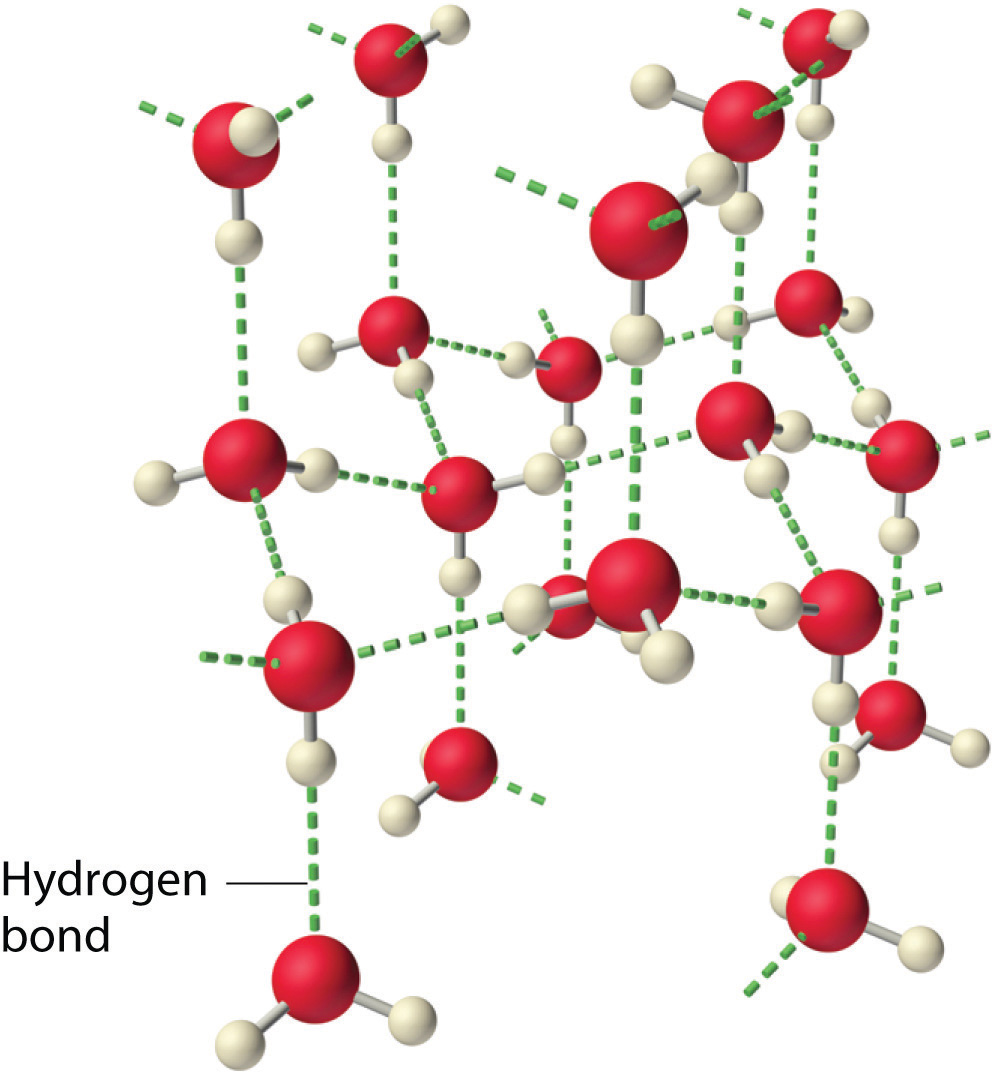
A hydrogen bond will form between the partially negativelycharged oxygen atom of one molecule and the partially positivehydrogen atom of another molecule. Water s large dipole moment leads to hydrogen bonding. When water molecules interact the hydrogen bonds pull the molecules together giving water and ice distinct properties.
Describe where a hydrogen bond can form among water molecules by signing up you ll get thousands of step by step solutions to your. These two liquids therefore mix together well and in just about any proportions. Although the molecule has a zero charge the charge is.
What is hydrogen bond. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. ø a single water molecule can form four hydrogen bonds with four different water molecules.
This is illustrated by the gradation in color in the schematic diagram here. The electronic negative charge is concentrated at the oxygen end of the molecule owing partly to the nonbonding electrons solid blue circles and. The h 2 o molecule is electrically neutral but the positive and negative charges are not distributed uniformly.
Water molecules forming hydrogen bonds with one another. Because of the attraction the water molecules can pull the solute molecules apart so that the solute dissolves in the water. ø it is a weak electrostatic attraction between a hydrogen and an electro negative atom.
Hydrogen bonding is responsible for surface tension and the crystalline structure of ice. Because the water molecules are small many of them can surround one molecule of the solute and form hydrogen bonds. Water molecules are also attracted to other polar molecules and to ions.
What are the properties of hydrogen bond. This occurs throughout a sampleof water. Hydrogen bonding is one of the strongest molecular forces second only to ionic bonding.
 Unit 2 Topic 2 Properties Of Water Ppt Download
Unit 2 Topic 2 Properties Of Water Ppt Download
Answer In General Chemistry For Jude 101935
 Hydrogen Bonding In Water Youtube
Hydrogen Bonding In Water Youtube
 Structure Of Water And Ice Biology Forums Gallery Quimica Universo
Structure Of Water And Ice Biology Forums Gallery Quimica Universo
 Lesson Summary Water And Life Article Khan Academy
Lesson Summary Water And Life Article Khan Academy
 Chapter 1 Molecules Of Life Ppt Download
Chapter 1 Molecules Of Life Ppt Download
 Hydrogen Bonds In Water Explained Intermolecular Forces Youtube
Hydrogen Bonds In Water Explained Intermolecular Forces Youtube
 Hydrogen Bonds Overview Examples Expii
Hydrogen Bonds Overview Examples Expii
 Structure Of Water Structured Water Digital Fabrication Hydrogen Bond
Structure Of Water Structured Water Digital Fabrication Hydrogen Bond
 Importance Of Water For Life Video Khan Academy
Importance Of Water For Life Video Khan Academy
 5 1 Properties Of Water Introduction To Oceanography
5 1 Properties Of Water Introduction To Oceanography
 11 9 Water An Extraordinary Substance Chemistry Libretexts
11 9 Water An Extraordinary Substance Chemistry Libretexts
 Why Life Depends On Water Biology For Non Majors I
Why Life Depends On Water Biology For Non Majors I
 Hydrogen Bonds What Are Hydrogen Bonds How Do Hydrogen Bonds Form Youtube
Hydrogen Bonds What Are Hydrogen Bonds How Do Hydrogen Bonds Form Youtube
 2 2 Properties Of Water Worksheet Answers Simplifying Algebraic Expressions Chemistry Lessons Worksheets
2 2 Properties Of Water Worksheet Answers Simplifying Algebraic Expressions Chemistry Lessons Worksheets

Komentar
Posting Komentar