Water Is Able To Form Hydrogen Bonds Because
The oxygen atom in a water molecule has a weak positive charge. 4 water is able to form hydrogen bonds because a oxygen has a valence of 2.
Https Www Dentonisd Org Cms Lib Tx21000245 Centricity Domain 6858 Unique 20properties 20of 20water 20ws 20 20answer 20key Pdf
The bonds that hold together the atoms in a water molecule are polar covalent bonds.

Water is able to form hydrogen bonds because. Each of the hydrogen atoms in a water molecule is weakly negative in charge. Water can form hydrogen bonds because of the polarity of its oxygen hydrogen bonds. D the oxygen atom in a water molecule has a weak positive charge.
The water molecule is shaped like a tetrahedron. Hydrogen bonds emerge due to the properties of a water molecule. C the bonds that hold together the atoms in a water molecule are polar covalent bonds.
Oxygen has a valence of 2. The water molecule is shaped like a tetrahedron. B the water molecule is shaped like a tetrahedron.
Each of the hydrogen atoms in a water molecule is. The bonds that hold together the atoms in a water molecule are polar covalent bonds. The oxygen atom in a water molecule has a weak positive charge.
Water is able to form hydrogen bonds because. The h of one water molecule and the o of another water molecule water is able to form hydrogen bonds because the bonds that hold together the atoms in a water molecule are polar covalent bonds. In these bonds oxygen has a partial negative charge and hydrogen has a partial positive charge.
Hydrogen atoms are able to bond with another water molecule due to it s polarity. Oxygen has a valence of 2. The water molecule is shaped like a tetrahedron.
A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. Water also has a high boiling point because of. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons.
Water is able to form hydrogen bonds because a. Oxygen has a valence of 2. The oxygen atom in a water molecule has a weak positive charge.
All of the electron pairs shared and unshared repel each other. The bonds that hold together the atoms in a water molecule are polar covalent bonds.
 Solved 4 Which Amino Acid Could Form Ionic Interactions Chegg Com
Solved 4 Which Amino Acid Could Form Ionic Interactions Chegg Com
 Lite Page 67 Water Is A Bent Molecule And This Allows It To Form Hydrogen Bonds Between Its Molecules What Are Three Ways In Which Your Life Would Be Different If Water
Lite Page 67 Water Is A Bent Molecule And This Allows It To Form Hydrogen Bonds Between Its Molecules What Are Three Ways In Which Your Life Would Be Different If Water
 Properties Of Water Biology Doodle Diagram Biology Lessons Biology Teaching Biology
Properties Of Water Biology Doodle Diagram Biology Lessons Biology Teaching Biology
 Our Water Molecule Comic Is Directly From Our Properties Of Water Video Https Youtu Be 3jwagwky98c Biol Teaching Chemistry Chemistry Lessons Science Humor
Our Water Molecule Comic Is Directly From Our Properties Of Water Video Https Youtu Be 3jwagwky98c Biol Teaching Chemistry Chemistry Lessons Science Humor
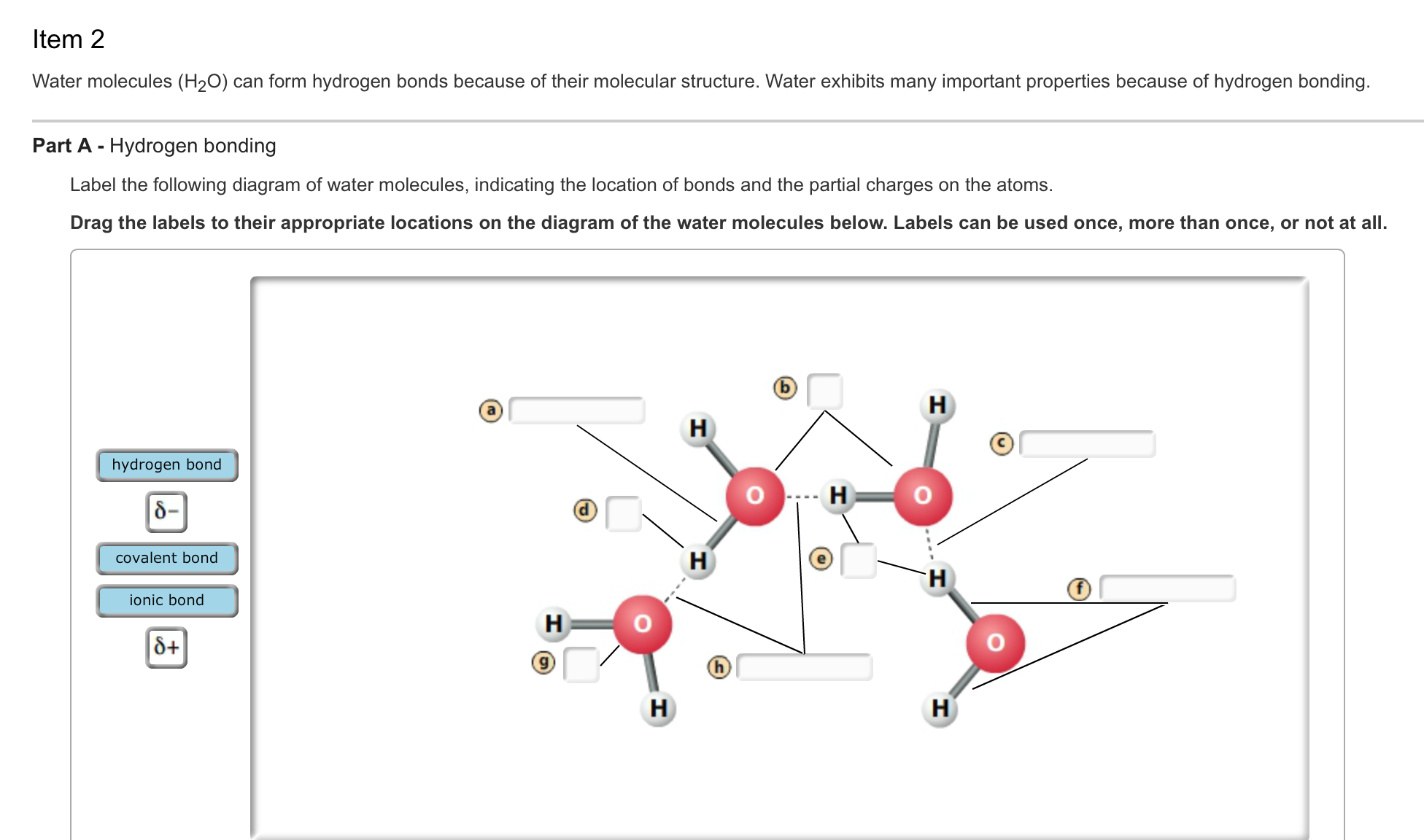 Solved Water Molecules H 2o Can Form Hydrogen Bonds Bec Chegg Com
Solved Water Molecules H 2o Can Form Hydrogen Bonds Bec Chegg Com
 Why Do H2o Molecules Form More Hydrogen Bonds Compared To Nh3 And Hf Molecules Youtube
Why Do H2o Molecules Form More Hydrogen Bonds Compared To Nh3 And Hf Molecules Youtube
 Liquids And Solids Chapter Ppt Download
Liquids And Solids Chapter Ppt Download
 Energy Levels Electrons And Covalent Bonding Teach Your Students About The Electrons Attractio Covalent Bonding Energy Level Teaching Middle School Science
Energy Levels Electrons And Covalent Bonding Teach Your Students About The Electrons Attractio Covalent Bonding Energy Level Teaching Middle School Science
 Hydrogen Bonds What Are Hydrogen Bonds How Do Hydrogen Bonds Form Youtube
Hydrogen Bonds What Are Hydrogen Bonds How Do Hydrogen Bonds Form Youtube
 Which Molecule Or Molecules Can Form Hydro Clutch Prep
Which Molecule Or Molecules Can Form Hydro Clutch Prep
 2 2 Properties Of Water Worksheet Answers Algebraic Expressions Simplifying Algebraic Expressions Chemistry Lessons
2 2 Properties Of Water Worksheet Answers Algebraic Expressions Simplifying Algebraic Expressions Chemistry Lessons
 In Liquid Water Individual Molecules Are Able To Move About At Any Given Time A Water Molecule Will Form An Average Of Hydrogen Bond Water Molecule Science
In Liquid Water Individual Molecules Are Able To Move About At Any Given Time A Water Molecule Will Form An Average Of Hydrogen Bond Water Molecule Science
 Pdf It Is Important To Compute Intramolecular Hydrogen Bonding In Drug Design
Pdf It Is Important To Compute Intramolecular Hydrogen Bonding In Drug Design
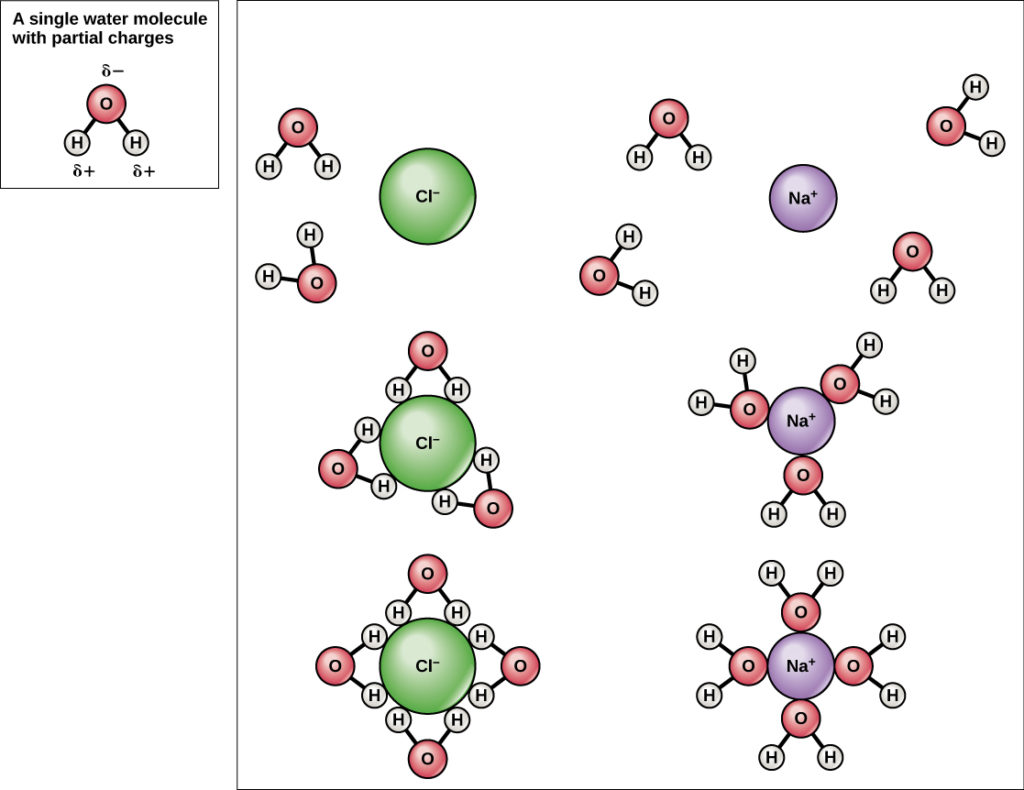 Why Life Depends On Water Biology For Non Majors I
Why Life Depends On Water Biology For Non Majors I
 Food Components In Food Sciences Basic Food Chemistry Ppt Download
Food Components In Food Sciences Basic Food Chemistry Ppt Download
Intro Bio Lec 2 Columbia University
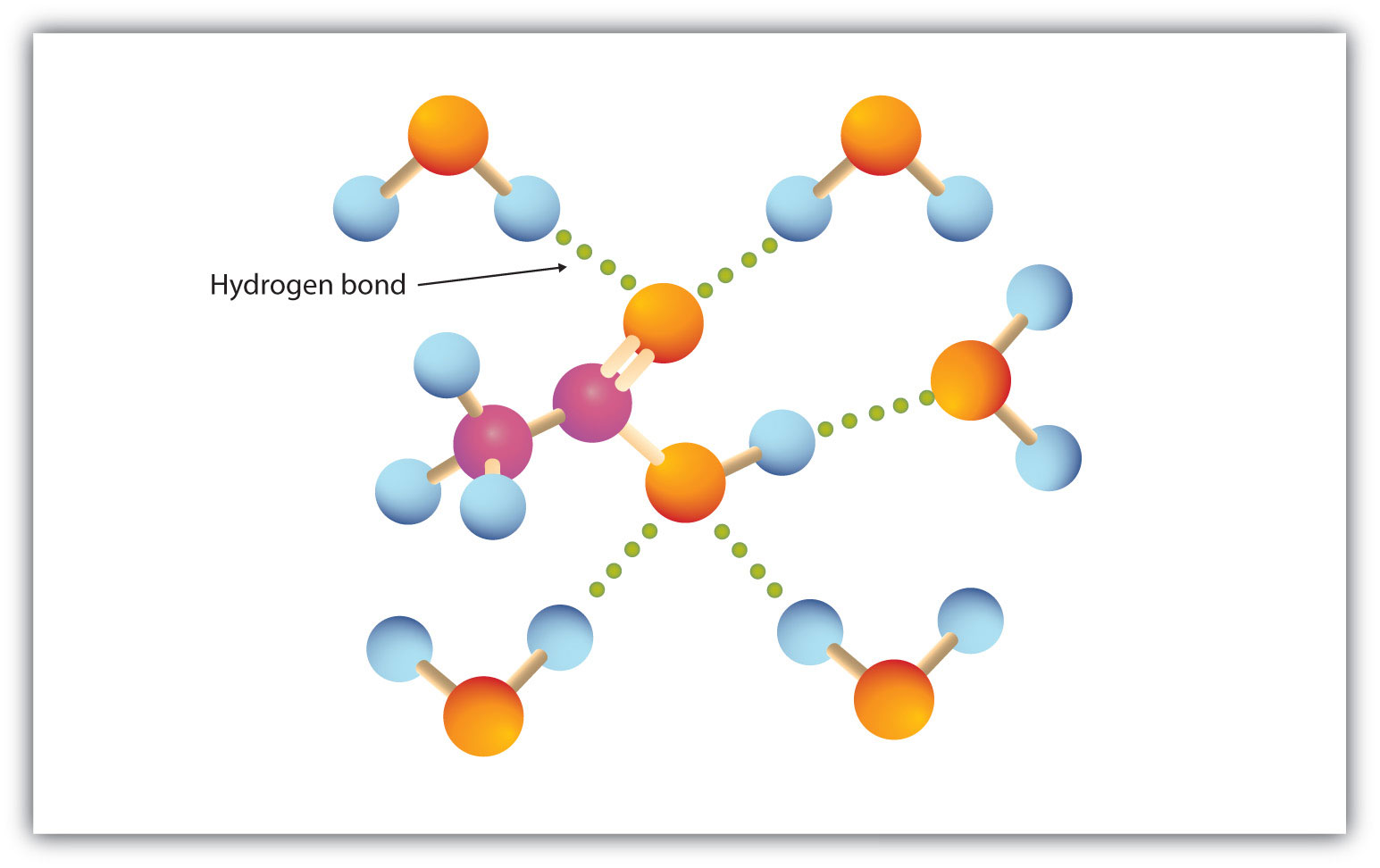


Komentar
Posting Komentar